Le projet est axé sur le développement d'outils technologiques de haut niveau combinant des approches biologiques, biomécaniques et d'imagerie des tissus au cours du processus de vieillissement.
Skin and cartilage tissue ageing
Leader Work package : H. Zahouani
Contacts locaux : S. Briançon, F. Mallein-Gerin, A.M. Sfarghiu, P. Sommer
Contacts cliniques : O. Damour, L. Thomas, T. Roger
Context and objectives
This project aims to study in a non invasive way the role of microstructures on the mechanical properties of skin and cartilage, and especially their evolution during ageing and repair. Recent advances in the biomechanics field highlight the need of further fundamental research to understand how this tissue assembles and acquires its unique properties.
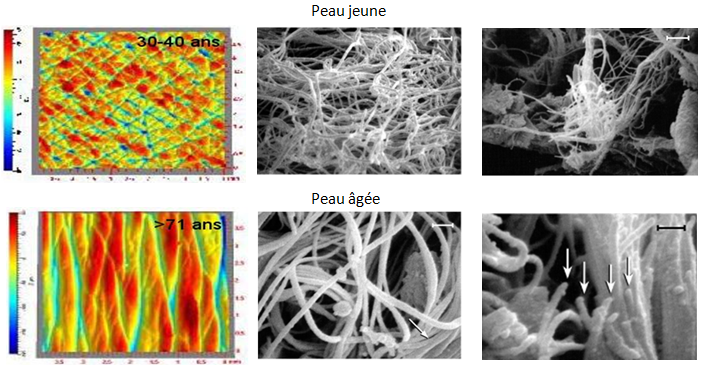
The development of new quantitative tools to identify their biomechanical properties is an essential step to increase knowledge in ageing soft tissue. This project at the frontier of mechanics and biology will benefit from the expertise in tissue biology and the most advanced experimental and modeling mechanical tools.
Biomechanical methods for following up the 3D elastic fibers network during ageing
During ageing and healing, several physical properties of tissues are lost. The extra cellular matrix (ECM) provides the structure of support, the elastic properties and resistance to the extension of tissues. The «elastic capital» degrades progressively with a speeded up manner in certain pathologies and are not replaced during healing and ageing.
To study the effect of the 3D network of elastic and collagen fibers during tissue ageing, we introduce the coupling of the multi-photon confocal microscope with a tensile test on ex vivo and in vitro models. The approach of 3D image correlation will enable to take into account the deformation of all the layers with the evolution of their visco-elastic properties, eventually as a function of normal or accelerated ageing.
In skin tissue, the challenge concerns the correlation between the deterioration of the network of elastic fibers and the macroscopic mechanical behavior. These results will be compared to in vivo morphometric index (wrinkles and printed lines).
Biomechanical properties for tissues interfaces
A fundamental feature of joints is their ability to take up and distribute high load and yet perform low friction movements. The articular cartilage covering the ends of the long bones facilitates their movement against one another and attenuates peak loads. With its dual function of bearing the load and providing a low friction interface, cartilage represents a key element in the joint. It is endowed with a remarkable dimensional stability and resistance to compression. On the one hand, the high osmotic pressure created by the negatively charged glycosaminoglycan chains of aggrecan opposes the applied stress. On the other hand, the high tensile stiffness of the cartilaginous collagen network provides the tissue with its tensile strength. Several studies using cartilage explants or chondrocytes seeded in three-dimensional (3D) scaffolds have shown that mechanical compressive loading does affect the chondrocyte metabolic activity.
However, little is currently known regarding mechanotransduction signaling in cartilage and new methods aimed to understand how mechanical force is converted into biochemical signaling need to be developed (see WP6). In addition, we need to understand how the mechanical properties of cartilage are affected by ageing and during the process of osteoarthritis, the degenerative pathology of cartilage. To do this, biophysical methods need to be adapted for cartilage studies, such as 3D multi-scale Morphoscan which gives access to mechanical and friction force with the resolution of the Atomic Force Microscope (AFM).
Skin composition and structure assessment by Confocal Raman Microspectroscopy
The molecular and structural composition of the skin and especially of its outermost layer is greatly affected by skin ageing and/or pathologies, involving changes in the skin barrier function, moisturizing, stratum corneum swelling or thinning.
Raman spectroscopy coupled with confocal microscopy is a powerful non invasive method, which can be applied to explore skin composition down to several hundreds micrometers below the skin surface. It will be used in vitro to generate information about skin samples molecular composition and structure (protein conformation, lipid packing) according to the skin type, age and possible disease; allowing to establish possible relationships between skin chemical composition and its mechanical properties.